Anti-Tri-Methyl Lysine Motif [tme-K] Antibody, Recombinant Antibody, R2
产品编号:RPTMC4A1-R2
¥ 询价
规格 100ug
 |
说明书 |
产品名称:Anti-Tri-Methyl Lysine Motif [tme-K] Antibody, Recombinant Antibody, R2
克隆号:R2
抗体亚型:/
经验证的应用:WB
交叉反应:All
特异性:Recognizes Tri-Methyl Lysine Motif [tme-K]
免疫原:Synthetic peptide
制备方法:/
来源:Recombinant Rabbit IgG
纯化:Protein A purified
缓冲液:Supplied in PBS, 50% glycerol and less than 0.02% sodium azide, PH7.4
偶联物:Unconjugated
状态:Liquid
运输方式:This antibody is shipped as liquid solution at ambient temperature. Upon receipt, store it immediately at the temperature recommended.
储存条件:This antibody can be stored at 2℃-8℃ for one month without detectable loss of activity. Antibody products are stable for twelve months from date of receipt when stored at -20℃ to -80℃. Preservative-Free. Avoid repeated freeze-thaw cycles.
图片:
Figure1.Western blot analysisof various cell lines,using RPTMC4A1-R2 at 2ug/ml dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) at 1:10000 dilution. Blocking buffer: 5% nonfat dry milk in TBST. Detection: ECL Basic Kit .
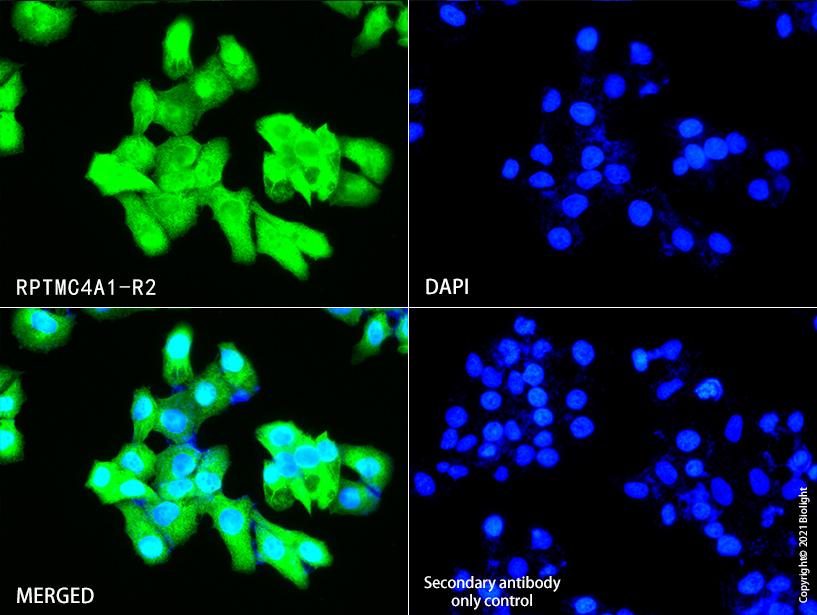
Figure2.Immunofluorescent analysis of Hela cells , using RPTMC4A1-R2 (green) at 10ug/ml dilution. Nuclear DNA was labelled in blue with DAPI (blue).
别称:/
背景信息:Methylation of lysine residues is a common regulatory posttranslational modification (PTM) that results in the mono-, di-, or tri-methylation of lysine at ε-amine groups by protein lysine methyltransferases (PKMTs). Two PKMT groups are recognized based on structure and catalytic mechanism: class I methyltransferases or seven β strand enzymes, and SET domain-containing class V methyltransferases. Both use the methyl donor S-adenosyl-L-methionine to methylate histone and non-histone proteins. Class I methyltransferases methylate amino acids, DNA, and RNA. Six methyl-lysine-interacting protein families are distinguished based on binding domains: MBT, PHD finger, Tudor, PWWP, WD40 repeat, and chromodomains. Many of these display differential binding preferences based on lysine methylation state. KDM1 subfamily lysine demethylases catalyze demethylation of mono- and di-methyl lysines, while 2-oxoglutarate-dependent JmjC (KDM2-7) subfamily enzymes also modify tri-methyl lysine residues.Most PKMT substrates are histone proteins and transcription factors, emphasizing the importance of lysine methylation in regulating chromatin structure and gene expression. Lys9 of histone H3 is mono- or di-methylated by G9A/GLP and tri-methylated by SETDB1 to activate transcription. JHDM3A-mediated demethylation of the same residue creates mono-methyl Lys9 and inhibits gene transcription. Tumor suppressor p53 is regulated by methylation of at least four sites. p53-mediated transcription is repressed following mono-methylation of p53 at Lys370 by SMYD2; di-methylation at the same residue further inhibits p53 by preventing association with 53BP1. Concomitant di-methylation at Lys382 inhibits p53 ubiquitination following DNA damage. Mono-methylation at Lys382 by SET8 suppresses p53 transcriptional activity, while SET7/9 mono-methylation at Lys372 inhibits SMYD2 methylation at Lys370 and stabilizes the p53 protein. Di-methylation at Lys373 by G9A/GLP inhibits p53-mediated apoptosis and correlates with tri-methylation of histone H3 Lys9 at the p21 promoter. Overexpression of PKMTs is associated with multiple forms of human cancer, which has generated tremendous interest in targeting protein lysine methyltransferases in drug discovery research.
全称:/
克隆号:R2
抗体亚型:/
经验证的应用:WB
交叉反应:All
特异性:Recognizes Tri-Methyl Lysine Motif [tme-K]
免疫原:Synthetic peptide
制备方法:/
来源:Recombinant Rabbit IgG
纯化:Protein A purified
缓冲液:Supplied in PBS, 50% glycerol and less than 0.02% sodium azide, PH7.4
偶联物:Unconjugated
状态:Liquid
运输方式:This antibody is shipped as liquid solution at ambient temperature. Upon receipt, store it immediately at the temperature recommended.
储存条件:This antibody can be stored at 2℃-8℃ for one month without detectable loss of activity. Antibody products are stable for twelve months from date of receipt when stored at -20℃ to -80℃. Preservative-Free. Avoid repeated freeze-thaw cycles.
图片:
Figure1.Western blot analysisof various cell lines,using RPTMC4A1-R2 at 2ug/ml dilution. Secondary antibody: HRP Goat Anti-Rabbit IgG (H+L) at 1:10000 dilution. Blocking buffer: 5% nonfat dry milk in TBST. Detection: ECL Basic Kit .
Figure2.Immunofluorescent analysis of Hela cells , using RPTMC4A1-R2 (green) at 10ug/ml dilution. Nuclear DNA was labelled in blue with DAPI (blue).
别称:/
背景信息:Methylation of lysine residues is a common regulatory posttranslational modification (PTM) that results in the mono-, di-, or tri-methylation of lysine at ε-amine groups by protein lysine methyltransferases (PKMTs). Two PKMT groups are recognized based on structure and catalytic mechanism: class I methyltransferases or seven β strand enzymes, and SET domain-containing class V methyltransferases. Both use the methyl donor S-adenosyl-L-methionine to methylate histone and non-histone proteins. Class I methyltransferases methylate amino acids, DNA, and RNA. Six methyl-lysine-interacting protein families are distinguished based on binding domains: MBT, PHD finger, Tudor, PWWP, WD40 repeat, and chromodomains. Many of these display differential binding preferences based on lysine methylation state. KDM1 subfamily lysine demethylases catalyze demethylation of mono- and di-methyl lysines, while 2-oxoglutarate-dependent JmjC (KDM2-7) subfamily enzymes also modify tri-methyl lysine residues.Most PKMT substrates are histone proteins and transcription factors, emphasizing the importance of lysine methylation in regulating chromatin structure and gene expression. Lys9 of histone H3 is mono- or di-methylated by G9A/GLP and tri-methylated by SETDB1 to activate transcription. JHDM3A-mediated demethylation of the same residue creates mono-methyl Lys9 and inhibits gene transcription. Tumor suppressor p53 is regulated by methylation of at least four sites. p53-mediated transcription is repressed following mono-methylation of p53 at Lys370 by SMYD2; di-methylation at the same residue further inhibits p53 by preventing association with 53BP1. Concomitant di-methylation at Lys382 inhibits p53 ubiquitination following DNA damage. Mono-methylation at Lys382 by SET8 suppresses p53 transcriptional activity, while SET7/9 mono-methylation at Lys372 inhibits SMYD2 methylation at Lys370 and stabilizes the p53 protein. Di-methylation at Lys373 by G9A/GLP inhibits p53-mediated apoptosis and correlates with tri-methylation of histone H3 Lys9 at the p21 promoter. Overexpression of PKMTs is associated with multiple forms of human cancer, which has generated tremendous interest in targeting protein lysine methyltransferases in drug discovery research.